Content uniformity and blend uniformity
Content uniformity and blend uniformity are the two most important tests which are performed for solid dosage forms like tablets, capsules and sachets. Many professionals are not aware of the key differences and basic information about blend uniformity and content uniformity...
Read more
Sterile and Non Sterile Gloves
This classification of disposable gloves is based on FDA (Food and Drug Administration) requirements & in this article, we will discuss the basic difference between the sterile and non-sterile gloves...
Read more
Alu Alu Vs Alu PVC Blister Packaging
Pharmaceutical packaging is necessary for dosage form protection and transportation. In this article, we will discuss the basic difference between the Alu Alu and Alu PVC packaging, which is used for the packaging of tablets and capsules in pharmaceutical industries...
Read more
Difference between LOD and Water Content
LOD & Water content methods are used in pharma quality control, where the water content method is used to measure the total amount of water present in a sample, and the LOD or moisture content method is used to measure the total amount of water and other volatile substances present in the sample...
Read more
Good Tablet Vs Bad Tablet
Have you ever thought about what is a good tablet and what is a bad tablet or defective tablet?...
Read more
Difference Between Lubricant and Glidant
Glidants increase the flow of powders or granules by reducing inter particle friction and lubricants reduce friction between product and machine parts...
Read more
Difference Between Pressure And Compound Gauge
Most people are aware of only the pressure gauge and they assume that there is only the pressure gauge that is used to measure all types of the vacuum and pressure but the reality is that there is a difference between pressure and vacuum gauge...
Read more
FDA Form 483 and warning letter
All the observations which are observed by the FDA officials during the on site inspection are noted on an official FDA document which is known as FDA form 483...
Read more
FIFO FEFO & LIFO In Pharma
The FIFO,FEFO & LIFO are three very common terms which are used for inventory management in raw material stores,warehouses & pharmacies...
Read more
Difference Between Classified & Unclassified Area
In this article we will discuss the examples of classified & non classified areas along with the key difference between classified & non classified areas...
Read more
Difference Between Equipment & Instrument
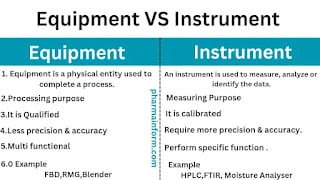
People working in pharmaceutical industries are frequently using the term equipment & instrument In their routine work & many of us think that both terms have the same meaning...
Read more
Difference Between QA & QC
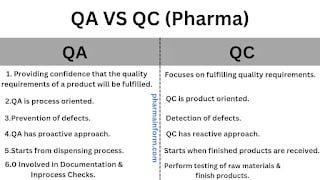
Everyone working in pharmaceutical industries is familiar with the term QA or Quality Assurance & QC or Quality Control but when we ask them to tell what is the difference ...
Read more
Difference Between Drug & Dosage Form
In this article, we are going to discuss one of the most common & confusing questions asked regarding the difference between drug and dosage form. The explanation will be very ...
Read more
Difference Between Wet Granulation & Dry Granulation
Granulation is the process that is largely used in pharmaceutical industries to manufacture Tablets, capsules, lozenges, pills & dry powder suspensions. Definition Of Granulation The process of converting fine powder ...
Read more
Difference Between Disintegration and Dissolution
Disintegration and dissolution are two most commonly used terms in pharma industries but most of the people do not know the exact difference between these two terms. In this article ...
Read more