Difference And Relation Between CPPs AND CQAs
Critical process parameters is an abbreviation of CPPs and CQAs is critical quality attributes. In this article we will learn what are the basic differences between critical process parameters and critical quality attributes and what is the relationship between these two...
Read more
CQA or Critical Quality Attributes
Chemical, physical, biological or microbiological characteristics or properties of a drug product must be controlled within a defined limit or range to ensure product quality...
Read more
Hold Time Studies In Pharma
WHO Technical Report Series (TRS) 992 annex 4 provides basic guide for hold time studies to the manufacturers & GMP inspectors...
Read more
Climatic Zones For Stability Studies
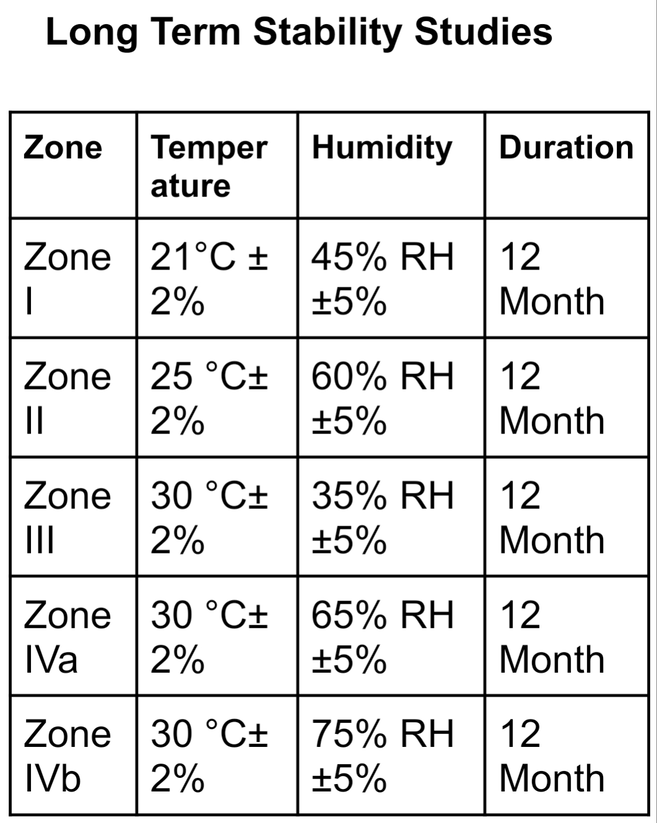
The world is divided into four climatic zones & zone IV is further subdivided into two zones which are IVa & IVb so by adding by division we have 5 ICH climatic zones...
Read more
Validation|Process validation
Validation is one of the most important and common terms used in pharmaceutical industries and its simple meaning is to provide a high degree of assurance or confirmation regarding product ...
Read more
Qualification|IQ|DQ|OQ|PQ|URS|FAT|SAT
Qualification is documented evidence that all the equipment, system, facility, or utility are designed, installed, and operated in such a way that complies with GMP requirements and produces products of ...
Read more
Difference Between Validation and Calibration
Validation is documented evidence that gives assurance that a process will consistently produce a product with pre-defined specifications and quality. Validation is documented proof that a process, procedure, or activity ...
Read more
Master Clock|Slave Clock In Pharmaceutical Industries
Time is a key factor for the success of any person or the organization.Time is also known as money and time can not be measured precisely without a clock or ...
Read more